How did ancient microbes help us process alcohol?

Fortunately for anyone who’s ever had a beer, a sip of wine, or a martini, humans are equipped with a biological process that breaks down alcohol. However, differences in our genetic code can impact this process and cause some people to experience symptoms of intoxication faster than others. One such difference occurs in an important gene that helps break down a byproduct of alcohol metabolism, known as the aldehyde dehydrogenase 2 (ALDH2) gene.
Alcohol causes intoxication and damage to both DNA and organs, yet in moderation we’re able to consume (and often even enjoy) it. How can that be? Actually, the proteins that make this possible have been used by life forms for billions of years1. Aldehyde dehydrogenase (ALDH) genes help our body convert toxic chemicals into less toxic byproducts. This was an essential ability for lifeforms billions of years ago as they were evolving, surrounded by highly reactive and toxic chemicals—sometimes even the byproducts of their own efforts to harvest energy. Consequently, nature developed numerous different members of the ALDH gene family. Each member is unique and able to interact with various types of molecules that are found in the environment, or in our body1.
But what does all of this have to do with alcohol metabolism? Of the 12 ALDH genes that humans have inherited, one in particular—ALDH2—works to recognize and eliminate a toxic byproduct that’s generated as our body breaks down alcohol. Because of this enzyme, humans are able to consume alcohol in small amounts without being poisoned.
Alcohol—more specifically, ethanol—is produced when certain microorganisms like yeast eat sugar. Surprisingly, humans and these microorganisms are not too different, at least in one regard—both use sugars to produce energy. When humans break down sugar, we generate both energy and lactate, which serve important roles in our physiology. Rather than generate lactate, some bacteria and yeast produce ethanol instead. You’re likely already familiar with this process, and just know it by a more familiar term: fermentation.
Fermentation often occurs naturally in ripe or rotting fruit. Ripe fruits in the wild are covered with yeasts that are consuming sugar and producing alcohol2. Both sugar and alcohol are very energy-rich molecules, which means our body can use them to produce energy (in the form of ATP). Because of this energy richness, animals seek ripe fruit2—but in order to consume it safely, they have to be able to digest the alcohol and prevent buildup of a toxic byproduct known as acetaldehyde. To do this, animals (including humans) rely on ALDH genes.
Alcohol metabolism can be described as a two-step process (see the figure below). The first step converts alcohol into the toxic byproduct acetaldehyde. The second step converts acetaldehyde into a less toxic chemical known as acetate. Interestingly, acetaldehyde may be the chemical responsible for the many effects that alcohol has on us3. When we drink alcohol, acetaldehyde begins to build up in our system which stimulates facial flushing, warming of the skin, rapid heart rate, and nausea. To prevent this, our body relies on the ALDH2 protein, which is mostly produced in the liver1,3. But some people experience facial flushing long before others do, and research into the genetics of alcohol metabolism have indicated that genetic variants may be to blame.

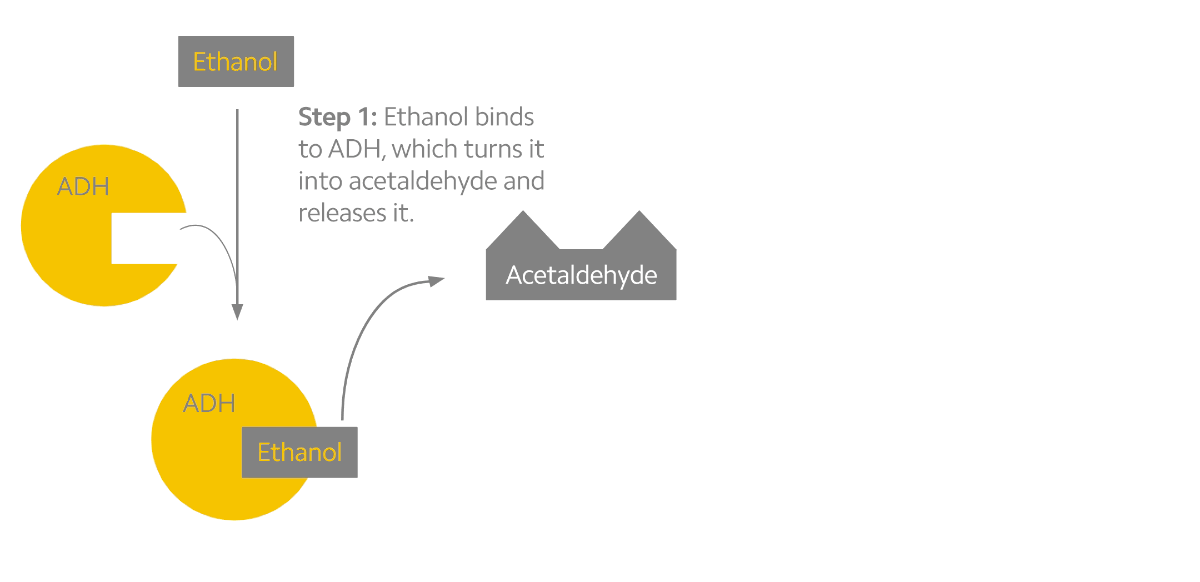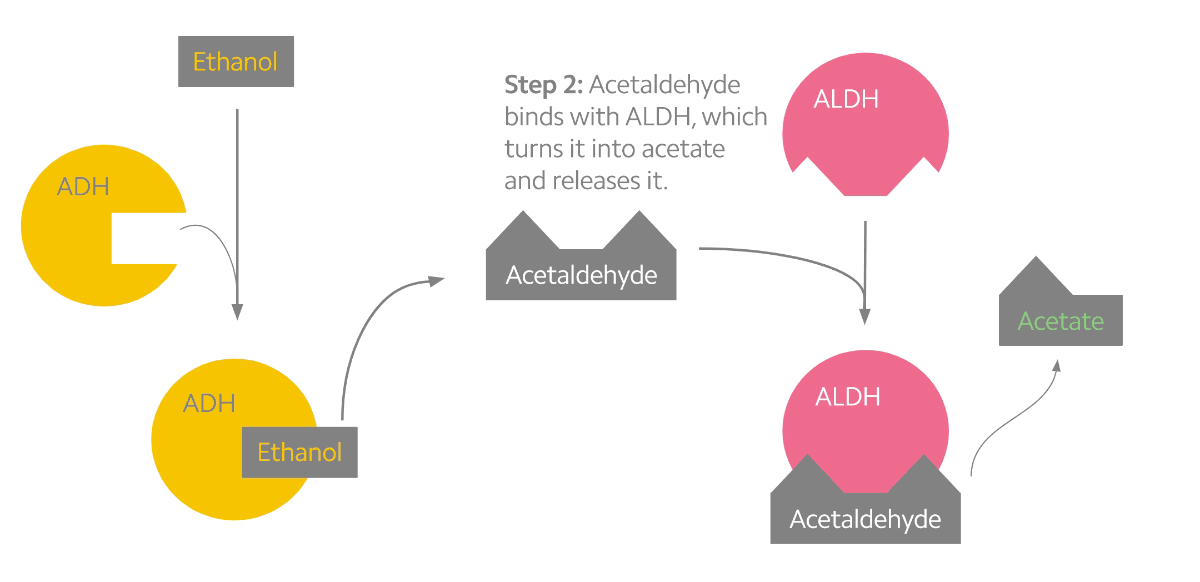
Alcohol metabolism in the body. Ethanol is converted to acetaldehyde by the ADH enzymes (yellow). Acetaldehyde is then rapidly converted to acetate by the ALDH enzymes (pink).
A single base change in the ALDH2 gene has been found to almost eliminate its ability to function4. Biologically, this means that altering the ALDH2 gene at this location significantly decreases the body’s ability to break down acetaldehyde into acetate. In response to a single drink, people who inherit this version of the ALDH2 gene (also known as the ALDH2*2 version) may accumulate higher levels of acetaldehyde than people without it3,4. Because of this, individuals with the ALDH2*2 variant are more likely to dislike alcohol3,4. (Regardless of what variants you do or don’t have in ALDH2, moderation is always important.)
Products in the Helix Store like Insitome’s Metabolism help you explore your ALDH2 gene and how it may be influencing your body’s response to alcohol. In addition to learning about specific genes, other products like Regional Ancestry can help you learn about the history that’s coded in your DNA by showing you the geographical regions where your ancestors may have lived.
And getting started with Helix couldn’t be easier: You just send in your sample using the DNA kit that comes with the purchase of Metabolism, Regional Ancestry, or other products in the Helix Store. Next, you’ll get your results and be ready to use your genetic data again with additional products, no new sample required. Are you ready? Get started today.
1Yoshida A, et al. “Human aldehyde dehydrogenase gene family.” European Journal of Biochemistry (1998): 10.1046/j.1432-1327.1998.2510549.x. Web. 24 Jan. 2018
2Dominy, Nathaniel J. “Ferment in the Family Tree.” Proceedings of the National Academy of Sciences of the United States of America 112.2 (2015): 308–309. PMC. Web. 24 Jan. 2018.
3Edenberg, Howard J. “The Genetics of Alcohol Metabolism: Role of Alcohol Dehydrogenase and Aldehyde Dehydrogenase Variants.” Alcohol Research & Health 30.1 (2007): 5–13. Print.
4Peng, Giia-Sheun, and Shih-Jiun Yin. “Effect of the Allelic Variants of Aldehyde Dehydrogenase ALDH2*2 and Alcohol Dehydrogenase ADH1B*2 on Blood Acetaldehyde Concentrations.” Human Genomics 3.2 (2009): 121–127. PMC. Web. 24 Jan. 2018.