Helix® Laboratory Platform Granted the First and Only FDA Authorization for a Whole Exome Sequencing Platform
FDA also clears the Helix® Genetic Health Risk App for Late-onset Alzheimer’s Disease for over-the-counter use, the first application powered by the Helix® Laboratory Platform.
Landmark market authorization of both a platform and its first test
Enabling Helix’s Sequence Once, Query Often™ model
Only FDA authorized WES platform
Broad coverage of the genome (~20,000 genes)
Separate clearances for platform and test
Enables new tests with minimal additional validation
Supports Rx and OTC test development
Pioneering a new regulatory pathway
Helix is proud to have collaborated with the FDA to pioneer a new regulatory pathway for the first-ever FDA authorized whole exome sequencing platform and its first application.
De Novo authorization
The HLP was granted FDA authorization through the De Novo pathway as a Class II device, resulting in a new regulation (21 CFR 866.6000) and product code (QNC) for a Whole Exome Sequencing Constituent Device (DEN190035)
510(k) clearance
The Helix® Genetic Health Risk App for Late-onset Alzheimer’s Disease was granted 510(k) clearance (K192073) to be used only with the Helix® Laboratory Platform
HLP analytical validation
Helix conducted analytical validation using a novel representative sampling-based approach in conjunction with rigorous quality metrics to establish accuracy and reproducibility of the device
Future test development
Helix and its partners are able to develop and clear future tests (such as for cancer, cardiovascular disease, and PGx) using data generated from the HLP - in most cases, without the partner needing to demonstrate additional analytical validity.

The Helix Laboratory
Data for the HLP is generated on the Exome+® assay in the Helix Laboratory, a high-complexity, CLIA-certified, CAP-accredited facility in San Diego, California. It is one of the highest-throughput clinical exome sequencing labs in the world. Helix has dedicated bioinformatics, laboratory, and quality teams ensuring the highest quality of data necessary to inform the broadest range of genetic insights.
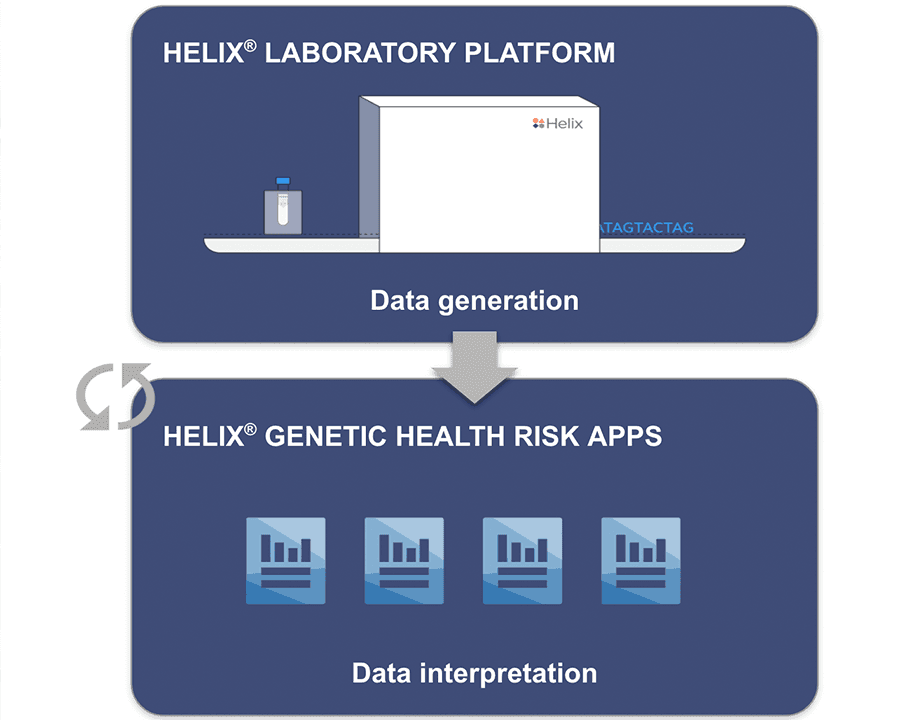
Supporting our Sequence Once, Query Often™ model
The parallel authorization of the Helix® Laboratory Platform and the clearance of the Helix® Genetic Health Risk App for Late-onset Alzheimer’s Disease demonstrates the significance of this advancement. Data generated on the Helix Laboratory Platform can be subsequently used to develop a myriad of genetic tests, enabling our Sequence Once, Query Often™ model.
The Helix® Laboratory Platform Intended Use
The Helix® Laboratory Platform is a qualitative in vitro diagnostic device intended for exome sequencing and detection of single nucleotide variants (SNVs) and small insertions and deletions(indels) in human genomic DNA extracted from saliva samples collected with Oragene®•Dx OGD-610. The Helix Laboratory Platform is only intended for use with other devices that are germline assays authorized by FDA for use with this device. The device is performed at the Helix laboratory in San Diego, CA.
The Helix® Genetic Health Risk App for Late-onset Alzheimer’s Disease Intended Use
The Helix® Genetic Health Risk App (HRA) uses qualitative genotyping to detect clinically relevant variants in genomic DNA isolated from human saliva collected from individuals ≥18 years with Oragene®•Dx OGD-610 for the purpose of reporting and interpreting Genetic Health Risks (GHR): The Helix Genetic Health Risk App (HRA) for late-onset Alzheimer’s disease is indicated for reporting of the e2/e2, e2/e3, e3/e3, e2/e4, e3/e4 and e4/e4 genotypes in the APOE gene. The report describes if a person's genetic result is associated with an increased or decreased risk of developing late-onset Alzheimer’s disease. The e2 and e4 variants included in this report are found and have been studied in many ethnicities. Detailed risk estimates have been studied the most in people of European descent. The Helix® Genetic Health Risk App (HRA) is to be used with the Helix® Laboratory Platform.