Technical Briefing 4: Severity of Acute Respiratory Illness
Executive summary
- Among patients with a medically attended respiratory virus infection, patients with SARS-CoV-2 or an endemic human coronavirus were less likely to be hospitalized (10%-11%) compared to patients with influenza, human metapneumovirus, rhinovirus, or RSV (58%-68%).
- Among patients with SARS-CoV-2 infection, prior receipt of a Pfizer-BioNTech or Moderna vaccine as one’s most recent SARS-CoV-2 vaccine dose was associated with a 60%-67% lower odds of hospitalization compared to being unvaccinated.
- Among patients with influenza infection, prior receipt of an influenza vaccine during the 2022-2023 influenza season was associated with an 85% lower odds of hospitalization.
Background
Real-world data studies conducted within healthcare delivery settings have played a crucial role in enhancing our understanding of health outcomes in individuals with medically attended respiratory virus infections. Such studies have provided valuable insights into the level of protection conferred by SARS-CoV-2 and influenza vaccines over time. Importantly, they have provided compelling evidence that both SARS-CoV-2 and influenza vaccines effectively guard against severe outcomes including hospitalization associated with SARS-CoV-2 or influenza infection and illness.
Leveraging data from health systems, we investigated differences in proportions hospitalized among patients infected with various respiratory viruses, lineages, and subtypes. Additionally, we evaluated the associations between prior SARS-CoV-2 or influenza vaccination and risk of hospitalization among individuals with medically attended SARS-CoV-2 or influenza infection.
Data source
Since February 2023, Helix has been collecting residual clinical samples from a healthcare system serving Minnesota and Wisconsin under an IRB-approved research protocol. These samples are from individuals who tested positive for a respiratory virus during diagnostic testing. In addition to the samples, Helix has obtained demographic metadata and information about the vaccination status of each infected individual, extracted from electronic health records and state vaccine registries.
This analysis included a total of 2,585 samples collected from patients between February 26, 2023 and June 5, 2023. The majority (2,333, 90%) were infected with SARS-CoV-2. Sixteen percent of samples (403) were collected among hospitalized patients, whereas the remaining 2,182 samples were collected in outpatient, urgent care, or emergency department settings. For this analysis, the type of visit associated with sample collection was the primary outcome (categorized as hospitalization versus outpatient, urgent care, or emergency department visit), serving as a surrogate measure of the severity of acute respiratory illness.
Proportions hospitalized by virus and lineage or subtype
Among patients with a medically attended respiratory virus infection, proportions hospitalized are displayed in Figure 1. A lower proportion of patients infected with SARS-CoV-2 (11%) or an endemic human coronavirus (HCoV) (10%) were hospitalized compared to patients infected with influenza (58%), human metapneumovirus (65%), rhinovirus (68%), or RSV (65%). Proportions were similar across SARS-CoV-2 lineages, influenza subtypes, and RSV subtypes. Among individuals with any of the three most common SARS-CoV-2 lineages in the U.S. (as of June 10, 2023), proportions hospitalized were 15% for XBB.1.5, 18% for XBB.1.16, and 18% for XBB.1.9.1. For influenza, 58% with subtype A(H1N1) and 53% with subtype B/Victoria were hospitalized. For RSV, 71% with RSV-A and 69% with RSV-B were hospitalized, although the number of patients with RSV was small.
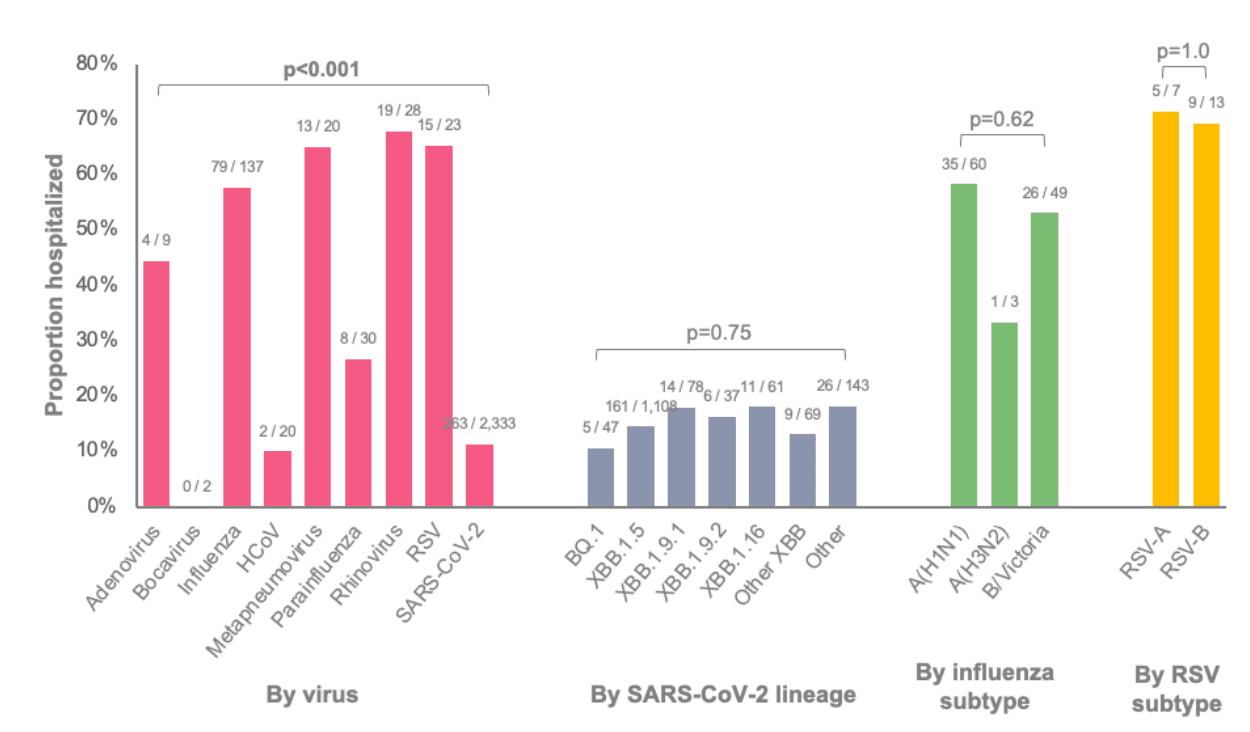
Figure 1: Proportions of patients with a medically attended respiratory virus infection who were hospitalized stratified by virus and lineage or subtype. Data for SARS-CoV-2 lineage and influenza or RSV subtype were restricted to patients with samples successfully sequenced.
SARS-CoV-2 vaccination and hospitalization among SARS-CoV-2 patients
Among patients with SARS-CoV-2 infection, unvaccinated patients were more likely to be hospitalized (15%) compared to patients who had been vaccinated since September 2022 (3%) or patients who were vaccinated but not since September 2022 (10%) (Figure 2). This difference was consistent among both adults and children.
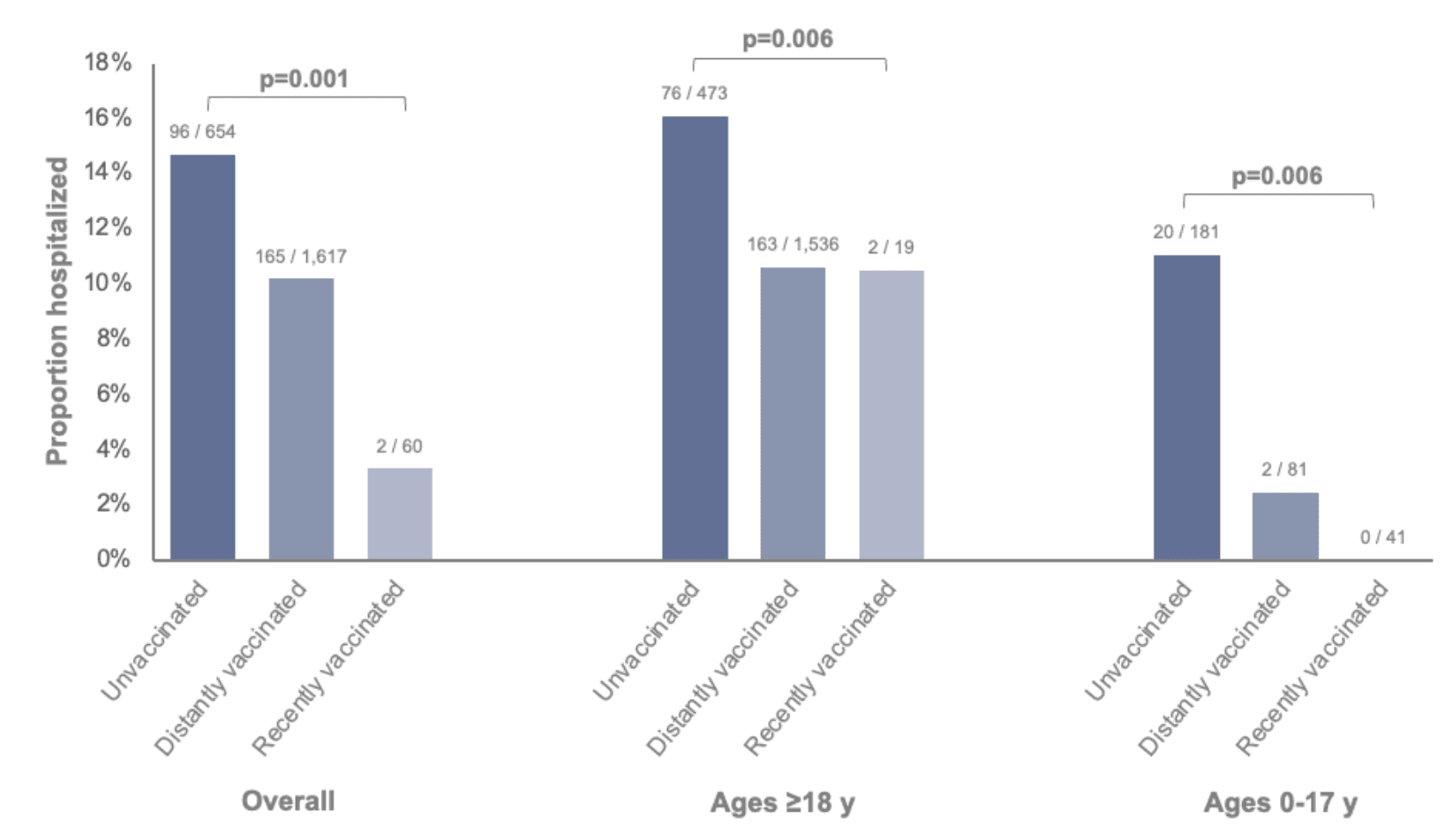
Figure 2: Proportions of SARS-CoV-2-positive patients who were hospitalized stratified by SARS-CoV-2 vaccination status and age group. Vaccination status was classified as unvaccinated (no dose ever documented), distantly vaccinated (last documented dose prior to September 1, 2022), or recently vaccinated (last documented dose after September 1, 2022). Bivalent mRNA vaccines were first recommended on this date as a booster dose for individuals aged ≥12 years (Pfizer-BioNTech) and ≥18 years (Moderna) who had previously received at least a primary series of any monovalent vaccine ≥2 months earlier, and have since been recommended for use as the primary series and as booster doses for individuals as young as 6 months of age. Two patients with their most recent SARS-CoV-2 vaccine dose within 0-6 days prior to the specimen collection date were excluded.
When classifying vaccinated patients based on the manufacturer of their last SARS-CoV-2 vaccine dose received, protection against hospitalization was similar for Pfizer-BioNTech and Moderna vaccine products (Figure 3). Receipt of a Pfizer-BioNTech vaccine as one’s most recent dose was associated with a 67% lower odds of hospitalization compared to being unvaccinated (adjusted odds ratio [OR]=0.33; 95% confidence interval [CI]: 0.24-0.47). Receipt of a Moderna vaccine as one’s most recent dose was associated with a 60% lower odds of hospitalization (adjusted OR=0.40; 95% CI: 0.26-0.59). These findings were observed despite a median of 447 days since patients’ last documented dose (interquartile range: 338-520), which was similar among Pfizer-BioNTech and Moderna vaccine recipients.
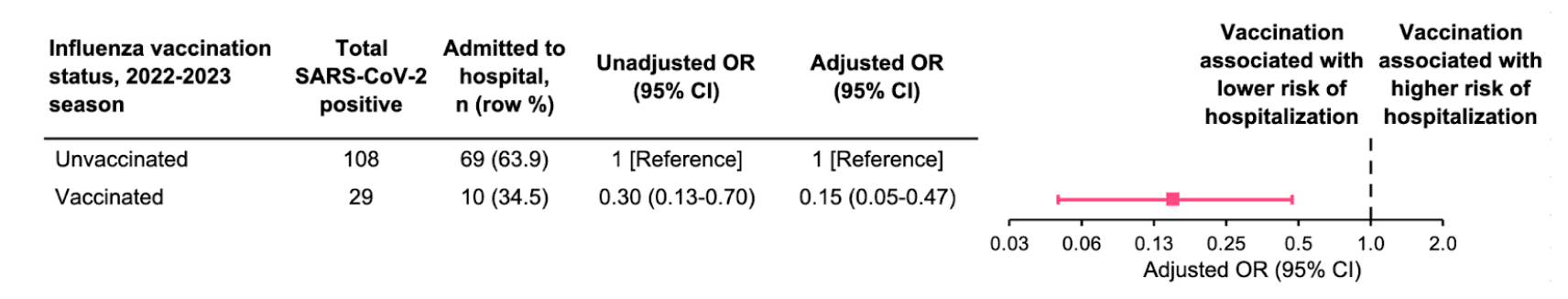
Figure 3: Association between last mRNA SARS-CoV-2 vaccine product received and hospitalization among SARS-CoV-2-positive patients. Analyses adjusted for age, sex, race/ethnicity, and specimen collection date. Age and specimen collection date were smoothed using natural cubic splines. Data are not shown for 30 Janssen vaccine recipients (three of whom were hospitalized), one Novavax vaccine recipient (not hospitalized), and 99 patients with unknown vaccine manufacturer (16 of whom were hospitalized).
Influenza vaccination and hospitalization among influenza patients
Among patients with influenza infection, unvaccinated patients were more likely to be hospitalized (64%) compared to vaccinated patients (34%) (Figure 4). In age-stratified analyses, this difference was observed among adults but not among children, which was a smaller subgroup composed of only 7 vaccinated patients.
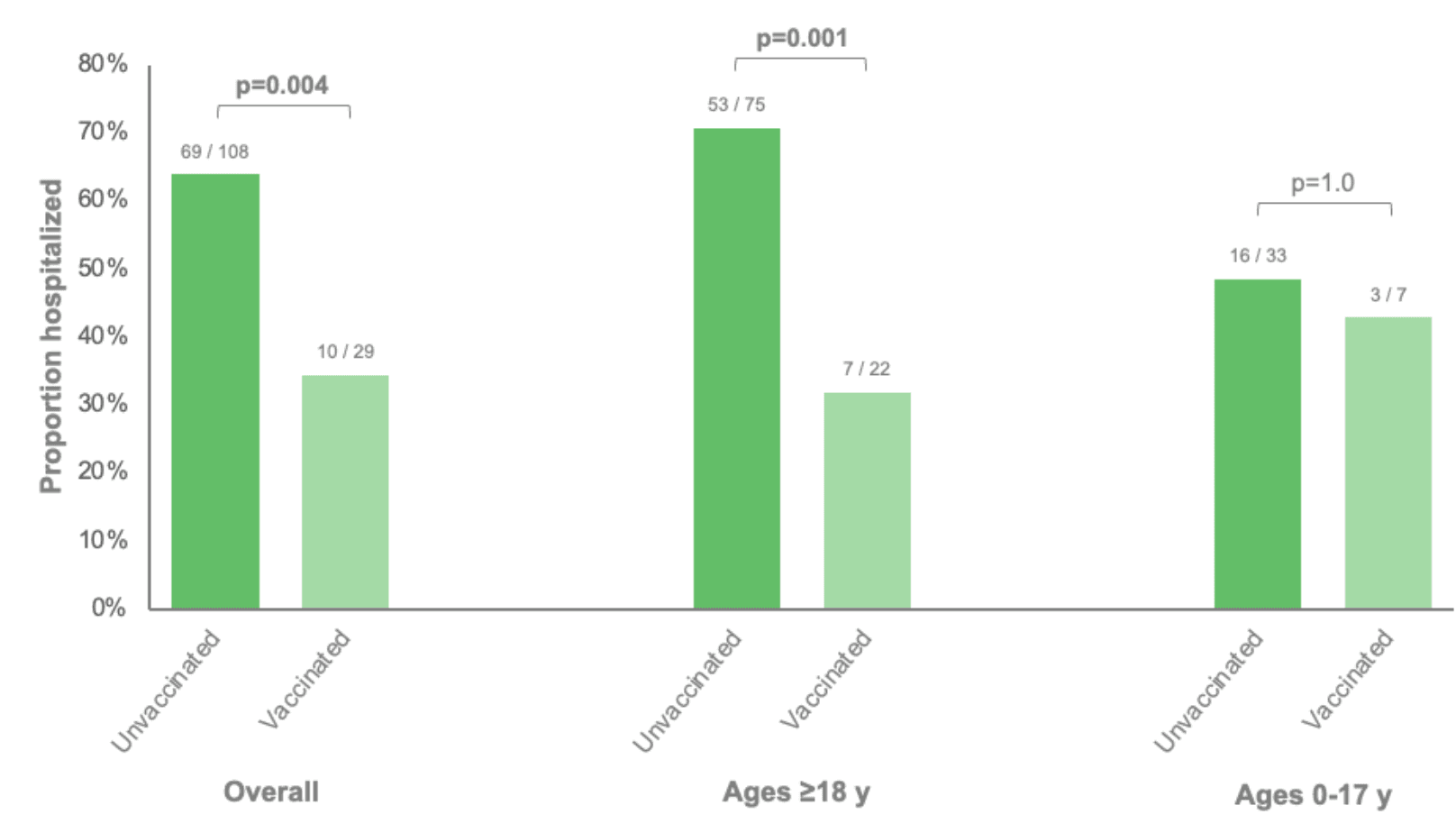
Figure 4: Proportions of influenza-positive patients who were hospitalized stratified by influenza vaccination status and age group. Vaccination status was classified as unvaccinated or vaccinated based on whether a dose was documented during the 2022-2023 influenza season. All vaccinated patients had been vaccinated ≥14 days prior to their specimen collection date.
Overall, influenza vaccination was associated with an 85% lower odds of hospitalization compared to being unvaccinated (adjusted OR=0.15, 95% CI: 0.05-0.47) (Figure 5).

Figure 5: Association between influenza vaccination status and hospitalization among influenza-positive patients. Analyses adjusted for age, sex, race/ethnicity, and specimen collection date. Age and specimen collection date were smoothed using natural cubic splines.
Caveats
Findings should be interpreted within the context of several limitations. First, documented diagnoses and symptomatology were not yet available for analysis, precluding our ability to characterize patients’ diagnoses, signs, and symptoms related to acute respiratory illness. We were also unable to account for the presence of underlying medical conditions including immunocompromising conditions, which may have differed between vaccinated and unvaccinated individuals. Second, whether patients were hospitalized was defined using the location and visit type associated with the specimen collected for respiratory virus testing, and was not evaluated longitudinally over the course of one’s infection or illness. Third, beyond SARS-CoV-2 vaccine manufacturer, the specific SARS-CoV-2 vaccine received (e.g., bivalent or monovalent) and type of influenza vaccine were not available for analysis. Misclassification of vaccination status is also possible if vaccination records were incomplete, which could have resulted in some patients who received SARS-CoV-2 vaccination since September 2022 having been classified as last vaccinated prior to then. Fourth, given that data for the current analysis was collected later in the 2022-2023 respiratory virus season, the numbers of patients infected with certain viruses, lineages, and subtypes were small-to-moderate, particularly when stratifying by age group and/or vaccination status.
The infrastructure of this study is evolving, with imminent plans to incorporate new network partners and expand the set of data elements collected from electronic health records. These advancements will largely address the current limitations and enable more comprehensive analyses in the future, particularly for investigations pertaining to the upcoming 2023-2024 respiratory virus season.
Prepared by Matt Levy and Shishi Luo.
© Helix, Inc. 2023. All rights reserved.
Helix and the Helix logo are registered trademarks of Helix, Inc.
Categories